Organic and Physical Chemistry

Isonitriles as Stereoelectronic Chameleons
Computational analysis of PhNC interaction with alkyl, aryl, heteroatom-substituted and heteroatom-centered radicals reveals a number of electronic supramolecular and conformational effects.
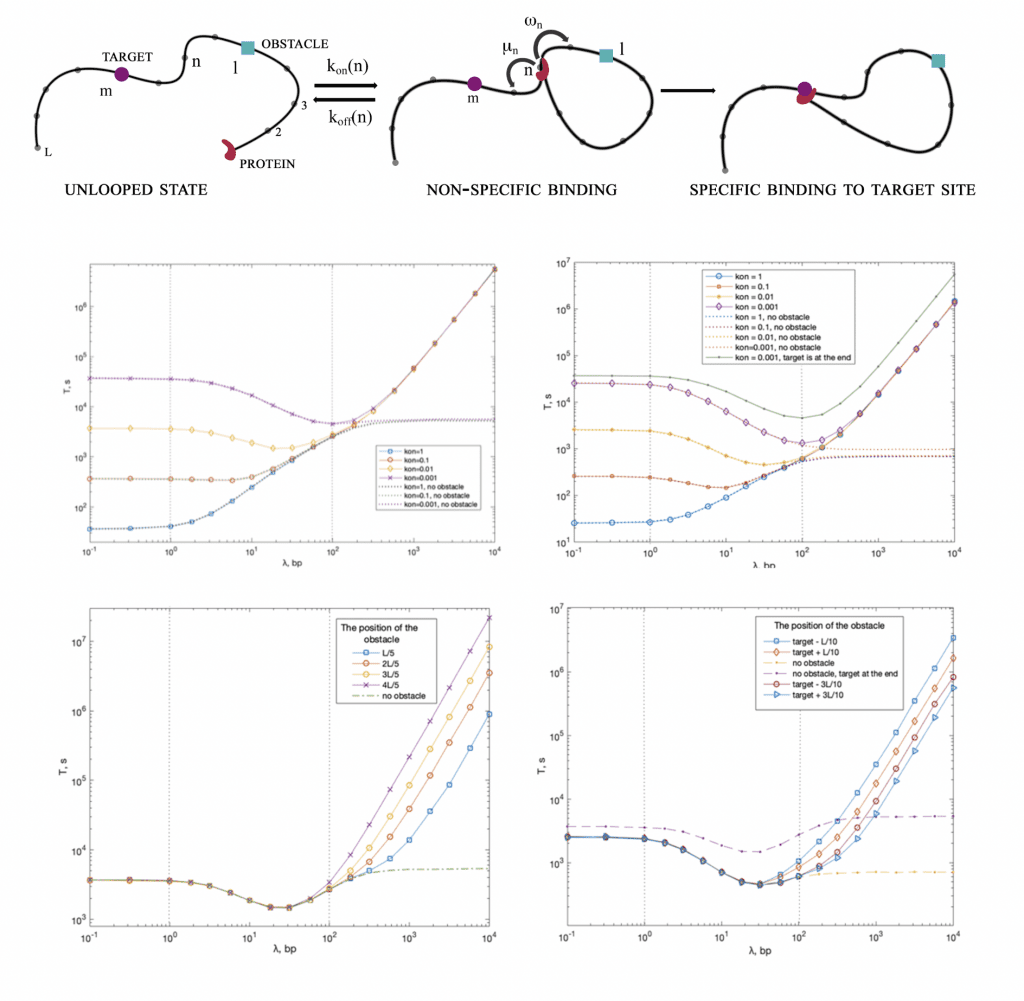
Protein Target Search
In this work, we develop a theoretical approach to evaluate the role of obstacles in the target search of multi-site proteins when the formation of DNA loops and the sliding in looped configurations are possible.
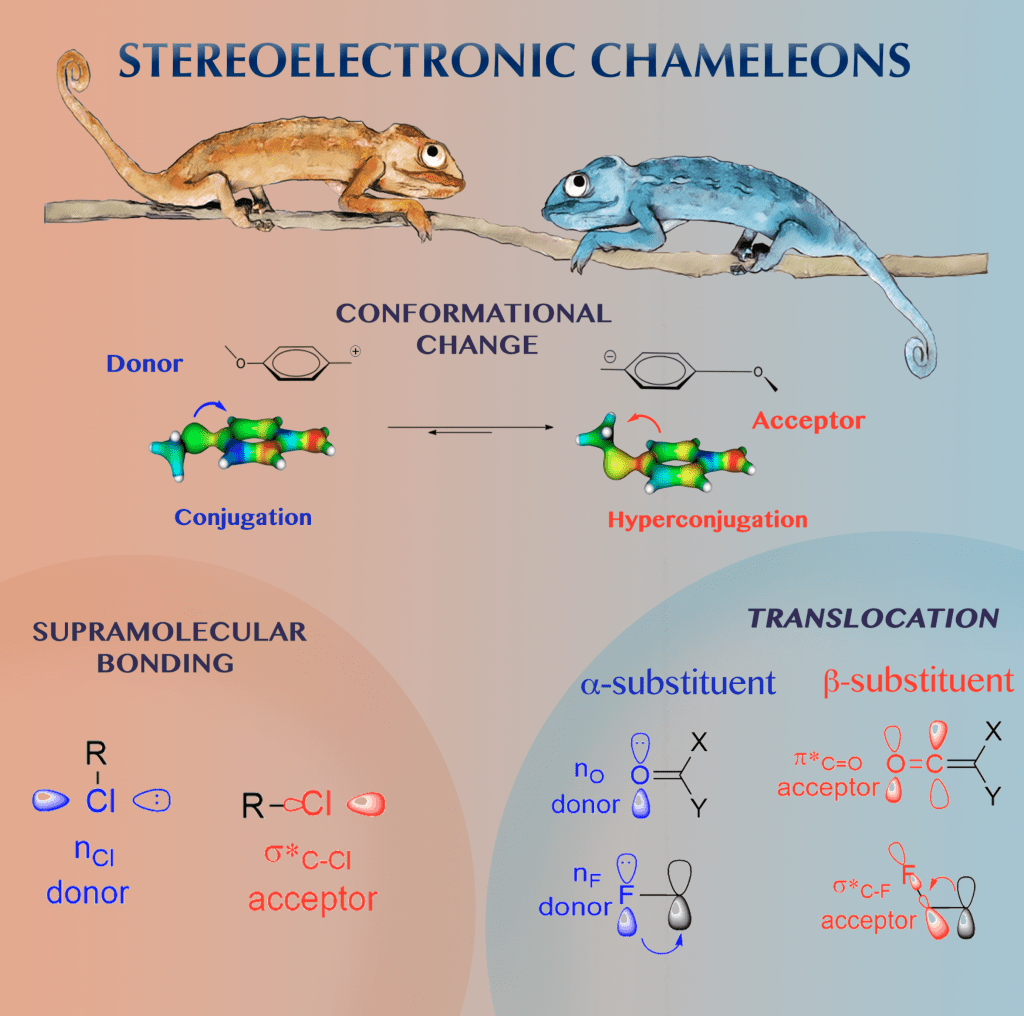
Stereoelectronic Chameleons
CHEMeleon: Stereoelectronic factors account for the apparent reversal of donor–acceptor properties of a variety of functional groups by a simple change of their orientation in space.
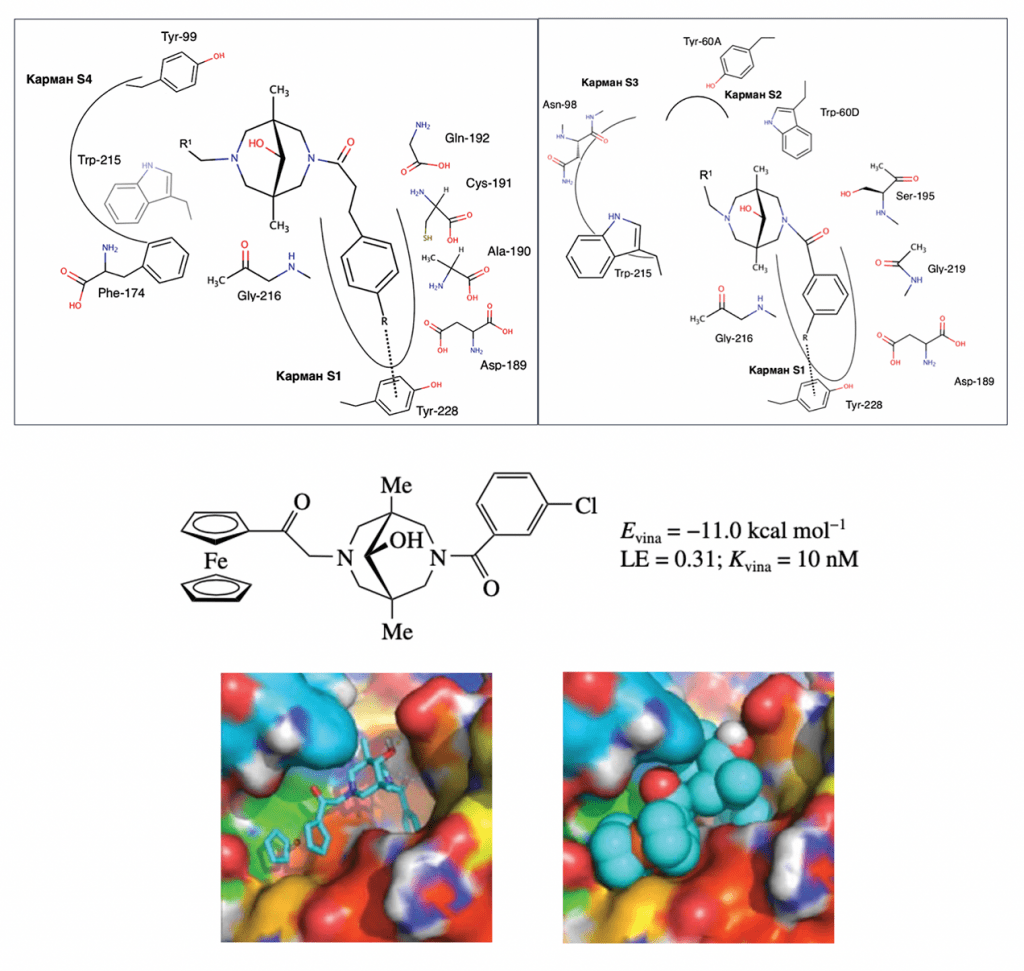
Serine protease inhibitors
The docking study of ferrocene-substitued bispidines to binding site of thrombine and factor Xa has shown that bispidine scaffold provides a 3D-arrangement of all substituents and a direction for the ferrocene group to fill the S4 pocket for both thrombin and factor Xa.
Website Development
Layered Lenses
Landing Pages
Malesuada fames ac turpis egestas integer eget aliquet nibh. maecenas pharetra convallis posuere morbi leo urna molestie at. Tellus rutrum tellus pellentesque eu tincidunt tortor aliquam nulla facilisi. alesuada fames ac turpis egestas integer eget aliquet nibh. Egestas maecenas pharetra convallis posuere morbi leo pharetra convallis posuere
Layered Lenses
Business Websites
Malesuada fames ac turpis egestas integer eget aliquet nibh. maecenas pharetra convallis posuere morbi leo urna molestie at. Tellus rutrum tellus pellentesque eu tincidunt tortor aliquam nulla facilisi. alesuada fames ac turpis egestas integer eget aliquet nibh. Egestas maecenas pharetra convallis posuere morbi leo pharetra convallis posuere
Personal Websites
electronic product prototyping
Malesuada fames ac turpis egestas integer eget aliquet nibh. maecenas pharetra convallis posuere morbi leo urna molestie at. Tellus rutrum tellus pellentesque eu tincidunt tortor aliquam nulla facilisi. alesuada fames ac turpis egestas integer eget aliquet nibh. Egestas maecenas pharetra convallis posuere morbi leo pharetra convallis posuere
Request additional information
Or Better Just Text me on
Telegram: @Julog
Email contact@company.com
